Prolonged-release tablets (1080 mg) used to dissolve stones in the urinary system.
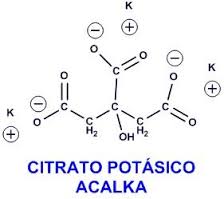
Classification
It belongs to the group of medicines called urinary concretion solvents that serve to dissolve the stones (stones) of the urinary system.
Acalka reduces the formation of calcium oxalate crystals and the precipitation of uric acid. Increases the pH of the urine trying to restore its normal levels without causing any alteration in the body.
It is indicated in the prevention and treatment of lithiasis in the kidney (formation of stones or stones in the kidney) due to calcium oxalate and / or calcium phosphate , from uric acid alone or accompanied by calcium stones and hypocitraturia (decrease in citrates in the urine) .
Knowledge before use
- If you are allergic (hypersensitive) to potassium citrate or any of the other ingredients of this medicine
- If you have kidney failure (kidney disease) as the use of Acalka could cause hyperkalaemia (increased potassium levels in the blood).
- If you have permanent alkaline urine infection.
- If you have obstruction in the urinary system (partial or total blockage of the urinary system that causes an interruption in the passage of urine through it).
- If you have hyperkalaemia (increased potassium levels in the blood).
- If you have adrenal insufficiency (disease of the adrenal glands leading to hormonal deficiency).
- If you have respiratory alkalosis (decreased levels of carbon dioxide in the blood) or metabolic alkalosis (increased levels of bicarbonate in the blood).
- If you have an active peptic ulcer (stomach or duodenal ulcer).
- If you have an obstruction in the intestine (partial or total blockage of the intestine that causes an interruption of the passage of the contents of the intestine through it).
- If you have slow gastric emptying (emptying of the stomach).
- If you are taking anticholinergic medicines.
Warnings
- Consult your doctor or pharmacist before taking Acalka.
- If you have liver failure (liver disease) or a decrease in the excretion (elimination by the body itself) of potassium since the use of Acalka could cause hyperkalaemia (increase in potassium levels in the blood).
- Potassium citrate is included in wax matrix tablets that allow slow release of potassium citrate. It is likely that once the waxy matrix is empty it will be eliminated intact by the depositions in a still visible way.
Using Acalka with other medicines
- Tell your doctor or pharmacist that you are using, have recently used or might have to use any other medicine.
- Acalka should not be taken together with potassium-sparing diuretic medicines (medicines used to increase urine output without increasing potassium output) such as Triamterene, Spironolactone, or Amiloride.
Using Acalka with food and drink
- While taking Acalka it is recommended to follow a salt-free diet and significantly increase your daily fluid intake.
If you are pregnant or breastfeeding
- If you are pregnant, take Acalka only if your doctor has told you to.
- Do not take Acalka if you are breast-feeding unless your doctor has told you to.
Driving and using machines
- The influence on the ability to drive or use machines is zero.
Recommendations for its use
- Follow exactly the instructions of administration of this medicine indicated by your doctor. If in doubt, check again.
Recommended dose
- Mild or moderate hypocitraturia (decrease in citrates in the urine):
The usual dose of Acalka is 1 tablet (10 mEq of potassium citrate) 3 times a day, 30 minutes after the 3 main meals.
- Severe hypocitraturia (decrease in citrates in the urine):
The usual dose of Acalka is 2 tablets (20 mEq of potassium citrate) 3 times a day, 30 minutes after the 3 main meals.
Not even very severe cases of hypocitraturia should not exceed 10 tablets. Acalka tablets are for oral administration.
- Swallow the tablets whole with enough liquid, without chewing, splitting, or dissolving in a liquid, 30 minutes after the 3 main meals to prevent minor gastrointestinal (stomach and intestinal) disorders.
Open the bottle following the instructions below: If you take more Acalka than you should
If you have taken more Acalka than you should, contact your doctor or pharmacist immediately. In case of overdose or accidental ingestion, also consult the Toxicology Information Service, telephone (91) 562.04.20. In the event of hyperkalaemia (increased potassium levels in the blood), treatment consists of administering an intravenous solution of 10% dextrose with 10-12 U of insulin / 1000 ml. If acidosis also occurs, treatment consists of intravenous sodium bicarbonate and hemodialysis or peritoneal dialysis.
If you forget to take any doses of the medicine
- Do not take a double dose to make up for a forgotten dose.
- If you have any further questions on the use of this medicine, consult your doctor.
Possible side effects
- Mild gastrointestinal (stomach and intestine) disorders that lessen if taken with food.
recommendations
- Keep this medicine out of the sight and reach of children.
- Keep the bottle tightly closed to protect it from light and moisture.
- Do not use this medicine after the expiry date which is stated on the carton and on the label after EXP. The expiration date is the last day of the month indicated.
- Medicines must not be disposed of via wastewater or household waste.
Composition
- The active ingredient is potassium citrate. Each tablet contains 1080 mg of potassium citrate (10 milliequivalents) which corresponds to 390 mg of potassium.
- The other ingredients (excipients) are: Carnauba wax (E-903) and Magnesium stearate.
What the product looks like and the contents of the pack
Presentation
- Prolonged-release tablets. It comes in packs of 100 tablets.
