The Third Law of Thermodynamics deals with the behavior of matter with entropy approximated to zero.
According to this law, whenever a system is in thermodynamic equilibrium, its entropy approaches zero.
The second law of thermodynamics relates to entropy. Subsequently, the third law appears as an attempt to establish an absolute reference point that determines entropy .
Walther Nernst (1864-1941) was the physicist who dealt with the principles that served as the basis for the third law of thermodynamics.
According to Nernst, entropy would tend to have a minimum value if the temperature of a pure substance were equal to or approached absolute zero.
For that, Nernst proposed the formula below, which shows that the entropy variation (ΔS) and temperature (T) tend to minimum values, that is, 0:
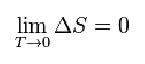
But, what is Entropy?
Entropy is how molecules organize themselves in the system. This organization translates into disorder, not in the sense of confusion, but in the sense of movement and agitation of molecules.
The more molecules can move, the more disorganized they are, the more entropy they have.
Initially, Nernst suggested that the entropy proposed by him would be possible only in perfect crystals.
Finally, he concluded that the temperature equal to absolute zero did not exist, which makes the third law a controversial law.
Thus, for many physicists, it is not a law, but a rule.
After so many years (since 1912), scientists are trying to obtain that temperature or temperatures that are getting closer and closer to absolute zero. Thus, they found that it is only possible in gases, discarding any substance in solid or liquid state.
