The saponification is a chemical reaction, also called triglyceride hydrolysis or alkaline hydrolysis of an ester, which occurs between an ester and an inorganic base.
The main source of esters, triglycerides, are vegetable oils and animal fats, widely used in this type of reaction.
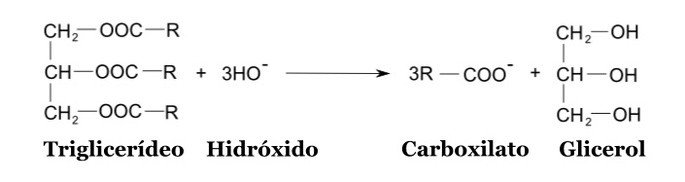
As products of the reaction, alcohol and organic salt of long carbon chain are formed, according to the general equation below.
Ester + Polyalcohol Base + Organic Salt
This organic reaction is mainly used to make soap, an organic acid salt, and glycerin, a tri-alcohol used as a humectant for soaps and beauty creams.
Saponification reaction example
In a simplified way, the structures of the compounds involved in this type of reaction are:
Below is an example of a saponification reaction.
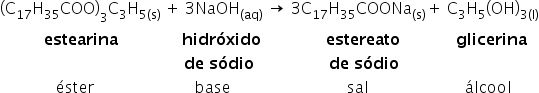
We have an example of a one-step reaction above using a strong base. However, there is also the possibility of carrying it out in two steps to obtain a better quality soap. Check out the saponification process:
Ester hydrolysis : formation of carboxylic acid and glycerin
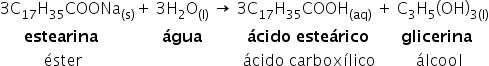
Neutralization of acid : there is the formation of the salt of carboxylic acid and water
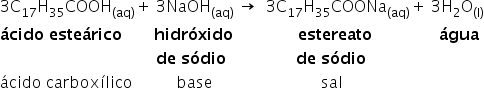
This type of reaction is exothermic, that is, the release of heat occurs in the formation of the products. The reverse reaction of ester hydrolysis is esterification.
Types of soaps produced with saponification
Depending on the base used, the characteristics of the soaps produced are changed, for example:
- Sodium soap: usually harder, this is the most common type;
- Potassium soap: soft, used in shaving creams;
- Ammonium soap: liquid, used in shampoos.
Soaps are used for cleaning due to their detergent action. The structure of these compounds is formed by a carbonic (apolar) chain, which interacts with fats, and an ionic (polar) end, capable of interacting with water and removing dirt in the wash.
Saponification index
The saponification index is the result of the reaction of the potassium hydroxide base (KOH) with one gram of oils or fats for complete saponification to occur.
See the table for the amount of base required to saponify some oils and fats.
| Triglyceride source | Saponification index (mg) |
|---|---|
| Fish oil | 189 to 193 |
| Swine Lard | 190 to 194 |
| Linseed oil | 190 to 195 |
| Chicken oil | 190 to 196 |
| Cotton oil | 190 to 200 |
| Bovine tallow | 190 to 202 |
| Butter | 210 to 235 |
History and importance of the saponification reaction
Since before Christ, Phoenicians and Romans had been carrying out saponification. Reacting goat fat with vegetable ash, under heating, the compounds sodium carbonate (Na 2 CO 3 ) and potassium carbonate (K 2 CO 3 ) present in the wood were able to saponify the triglycerides.
Due to the concern with personal hygiene, saponification has become increasingly important and soaps have for a long time been manufactured at home, using caustic soda (NaOH).
New technologies have allowed the manufacture of soap in other ways, for example, using water, instead of an inorganic base, under high temperature in equipment called autoclaves.
The saponification reaction also occurs within the human body. Bile is a substance released at the beginning of the small intestine to prevent the decomposition of the bolus, because it saponifies fats.
