Methanol or methyl alcohol is an organic compound of the family of alcohols, whose molecular formula is CH 3 OH (the same as CH 4 O).
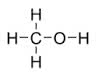
Also known as carbinol, it is liquid, colorless, water-soluble, toxic, highly flammable and with an almost imperceptible flame.
Its boiling point is reached at 65 ºC, while its melting point is reached at -98 ºC.
Obtaining
Methanol can be obtained through the distillation of wood, the reaction of a mixture of gases (reaction of the synthesis gas) or, still, of sugar cane.
Initially it was obtained only by the distillation of wood, which is why it became known as wood alcohol.
properties
- Highly flammable
- Toxic
- Soluble in water
- Polar solvent
- Boiling at 65ºC
- Fusion at -98 ºC
- Density: 792 kg / m 3
- Molar mass: 32.04 g / mol
applications
Methanol is used primarily as a solvent in the pharmaceutical industry.
It is also used as fuel for race cars and jet planes. This compound is used in the production of biodiesel and plastics and, finally, in the extraction of products of animal and vegetable origin.
Methanol and Ethanol
Methanol and ethanol are the main alcohols that exist. Both are combustible and, although methanol is more economical, it causes corrosion in steel and increases the chance of accidents due to the fact that its flames are almost invisible.
In polluting terms, both have the advantage of not producing sulfur dioxide (SO 2 ).
Another difference is that, while ethanol can be ingested, ingestion of methanol can be fatal or cause serious sequelae, such as blindness.
Remember that ethanol is used to make alcoholic beverages.
