Helium is the He symbol element of the Periodic Table, which has the atomic number 2, and is found under ambient conditions in the form of a gas.
The main characteristics of helium are: monatomic gas, low weight, colorless, odorless, non-flammable and non-toxic.
It is an element abundant in the mass of the sun and stars. On Earth, it is found next to natural gas, in addition to being produced by the disintegration of other elements.
The amount of helium gas available on the planet is sufficient for it to have different uses, ranging from inflating balloons to use in diving equipment.
Helium properties
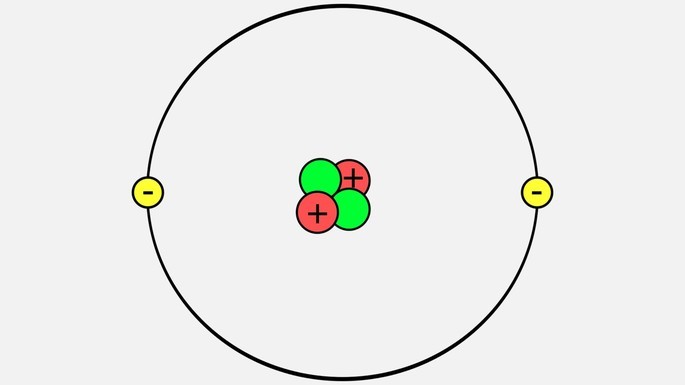
- Atomic Number: 2
- Molar mass: 4.0026 g / mol
- Electronic distribution: 1s 2
- Density: 0.0001785 g / cm 3
- Melting point: – 272.12 ºC
- Boiling point: – 266.934 ºC
- Physical state: gaseous in CNTP (Normal Temperature and Pressure Conditions)
Helium gas applications
Probably the best known application of helium gas is to use balloons . Because they have a lower density than air, helium balloons float when released. This application is not restricted to decorative balloons, but is also useful for air balloons and weather balloons.

In medicine, helium gas is used to treat obstructive diseases of the respiratory system, such as asthma and bronchiolitis. In the respiratory tract, a mixture of helium and oxygen can improve ventilation in the alveoli, facilitate the diffusion of carbon dioxide and decrease respiratory pressure.
Helium gas is inserted into the mixture of air cylinders for diving to avoid nitrogen narcosis, an effect similar to drunkenness, caused by the dilution of nitrogen in the blood of divers.
Discovery of helium
Its name comes from the Greek helios , which means sun. It is the second most abundant chemical element in the universe, which was first observed in the solar chromosphere during an eclipse in 1868 by astronomers Pierre Janssen and Norman Lockyer.
The spectrum produced by helium had a yellow color, unlike anything known at that time and, therefore, they deduced to be a new chemical element.
Later, in 1895, the radiation emitted by helium was observed in a uranium ore, the cleveite, studied by William Ramsay and confirmed by Lockyer through the spectrum produced.
At the same time, Per Cleve and Nils Abraham Langlet, while also studying the ore, made a spectroscopic identification of helium.
With the organization of the Periodic Table, helium was inserted in the family of noble gases, group 18, due to its low reactivity and to be considered practically inert.
Helium facts
- In the Sun, when two hydrogen atoms fuse, the chemical element helium originates and energy is produced.
- Helium is the only element in the noble gas family that lacks 8 electrons in the valence shell.
- At absolute zero, 0 K or – 273 ºC, helium is the only element capable of remaining in a liquid state.
- When inhaled, helium gas causes the voice to speak to be thinner than normal, as it increases the frequency of the sound.
